Blockchain startup Chronicled has announced the official launch of its MediLedger Product Verification Solution.
The solution aims to assist drug supply chain stakeholders in ensuring compliance with the saleable returns requirements of the Drug Supply Chain Security Act (DSCSA). The launch comes ahead of the regulatory deadline in late November 2019.
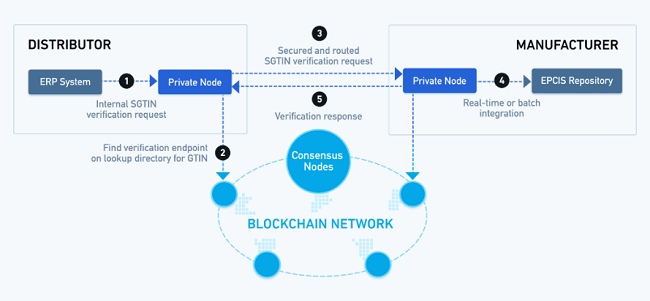
MediLedger Product Verification is an output of the MediLedger Project, which aims to create an open and decentralized network for the pharmaceutical supply chain. It has been developed in collaboration with leading pharma manufacturers, wholesale distributors, and innovative industry solution providers.
The objective was to create a product verification ecosystem which based on open standards, blockchain hosted look-up directory, and encrypted peer to peer messaging, allowing pharma industry players to verify the authenticity of prescription medicines as required by DSCSA.
Chronicled said that the launch marks an important step in its plans to address the pharma industry’s most challenging problems using the MediLedger Blockchain Network. It added that it has planned additional protocols which will allow compliance with upcoming DSCSA requirements and facilitate significant cost-savings in the industry's revenue management processes.
"Our industry has come together over the last 18 months to define, build, and test the MediLedger Product Verification Solution. We are especially thankful for the collaboration of the incumbent solution providers who are hosting and operating this truly decentralized blockchain network. We believe this is only the beginning of industry problems that can be solved using this innovative technology,” Susanne Somerville, CEO at Chronicled, said.
Last month, the MediLedger Project, which is coordinated by Chronicled, commenced its participation in the U.S. Food and Drug Administration (FDA) Pilot Project Program focused on developing a digital, interoperable system to help identify and trace certain prescription drugs as they are distributed within the United States.
Earlier in May, industry giants including Pfizer, McKesson Corporation, AmerisourceBergen Corporation, and Premier joined MediLedger Project Contracting and Chargebacks working group.























Comment 0